



















Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
A comprehensive overview of natural and synthetic rubbers, exploring their properties, production processes, and applications. It delves into the structure and characteristics of natural rubber, including its source, composition, and limitations. The document then explains the process of vulcanization, highlighting its importance in enhancing the properties of natural rubber. It also discusses various types of synthetic rubbers, such as styrene rubber and nitrile rubber, outlining their unique properties and applications. Valuable for students studying polymer chemistry, materials science, or engineering.
Typology: Summaries
1 / 27

This page cannot be seen from the preview
Don't miss anything!




















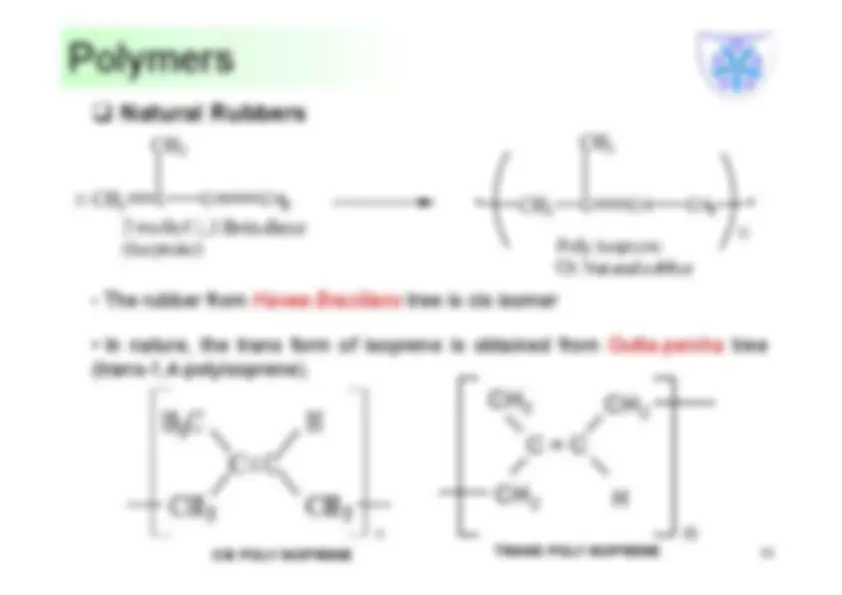
their length and return to their original form after removing the stretched force. Because the carbon chains are not in straight chained they are in the form of coils. Rubber is used to describe a material with elastic properties. It can be both natural and synthetic. Elastomers due to inter molecular forces attains a coiled amorphous structure, when a stress is applied, deformation starts by breaking the weak inter molecular force and attains a linear ordered structure. When the stress is taken back, the material regains the coiled structure and becomes amorphous once again.
Basically an elastomer is a polymer with very weak inter molecular network structure / polymers that are joined by chemical bonds with slightly crosslinked structure. Natural rubber is thermoplastic and it becomes thermoset and/or elastomeric depending on the extent of crosslinking (Vulcanisation).
•Commercial source of natural rubber is from the latex of para rubber tree – Hevea Brasiliensis. •The main constituent of the rubber latex will be poly cis-1,4 isoprene (2-methlybuta-1,3- diene) which will be around 30%-40% in the form of colloidal solution in water. •It is an addition polymer, addition between molecules of isoprene takes place by 1, addition and one double bond shifts between 2&3 positions.
76
•Latex is a suspension of microscopic natural rubber particles in aqueous medium. The surface of the latex particles are charged and this creates a force of repulsion which keeps them away from coalescing. •Rubber is from the latex mixture, by coagulating with acid (Acetic acid or Formic acid). Acid neutralizes the charge between the particles and forms a homogeneous precipitate.
81
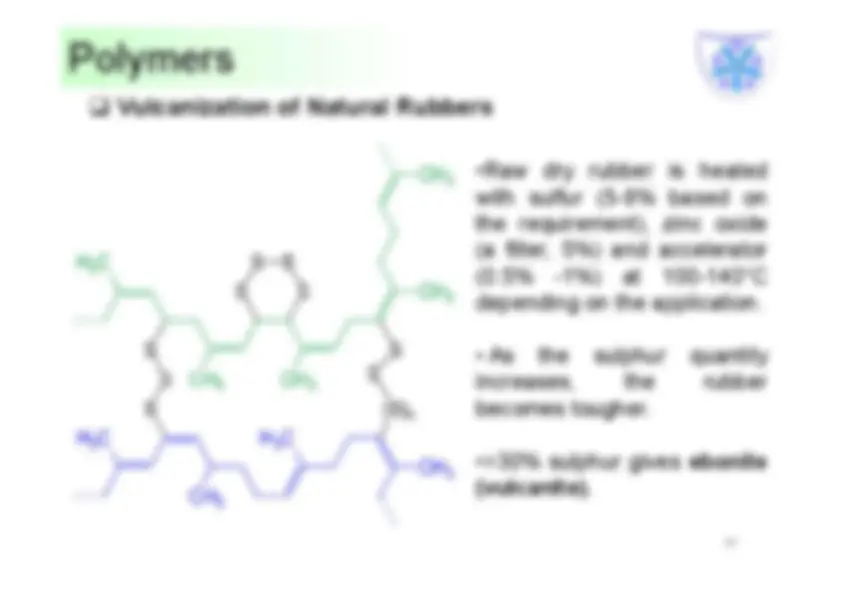
•Raw dry rubber is heated with sulfur (5-8% based on the requirement), zinc oxide (a filler, 5%) and accelerator (0.5% -1%) at 100-140°C depending on the application.
Advantages of vulcanization:
Raw Natural Rubber Vulcanized Natural Rubber Soft and sticky Comparatively hard and non-sticky Low tensile strength and not very strong (200Kg/cm 2 ) High tensile strength and very strong (2000Kg/cm 2 ) Can be used over a narrow range of temperature from 10 to 60 °C Can be used over a wide range of temperature from -40 to 100 °C Low abrasion resistance High abrasion resistance Absorbs a large amount of water Absorbs a small amount of water Soluble in solvents like ether, carbon disulphide, carbon tetrachloride, petrol and turpentine Insoluble in all the usual solvents Chemical resistance is low Chemical resistance is high Tackiness is high Tackiness is low.
Styrene rubber (Buna-S or Styrene Butadiene rubber SBR or GR-S)
Styrene rubber (Buna-S/SBR or GR-S)